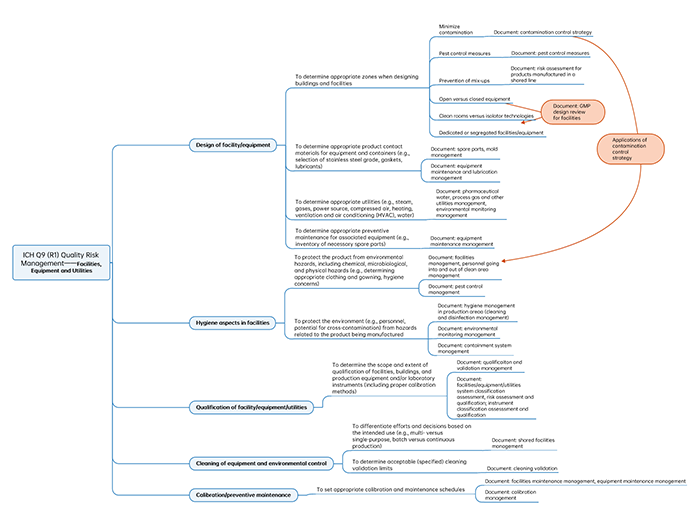
A Figure Illustrating QRM Applications and Implementation Documents of ICH Q9 (R1) Specific to Facilities and Equipment
News & Insights2024-09-19
Facilities and equipment, as the essential hardware for drug production, are an integral part of the pharmaceutical quality system (PQS). The design, use, cleaning, disinfection and maintenance of facilities and equipment will have a significant impact on the drug quality.

The following figure is the topic to illustrate: QRM Applications and Implementation Documents for Facilities and Equipment.
For HD reference, please click the link:
 A Figure Illustrating QRM Applications and Implementation Documents of ICH Q9 (R1) Specific to Facilities and Equipment.png
A Figure Illustrating QRM Applications and Implementation Documents of ICH Q9 (R1) Specific to Facilities and Equipment.png







 Search
Search 中文
中文







