Analysis on Correlation of Equipment Lifecycle Management Process with EU GMP Annex 1
News & Insights2024-08-15
EU GMP Annex 1 states:
Contamination control strategy encompasses elements such as facilities and equipment, utilities, supplier approval, preventative maintenance and cleaning and disinfection.
Section 5 Equipment provides general guidance on the design and operation of equipment.

This article employs the following figure to illustrate the correlation of equipment lifecycle management process with EU GMP Annex 1:
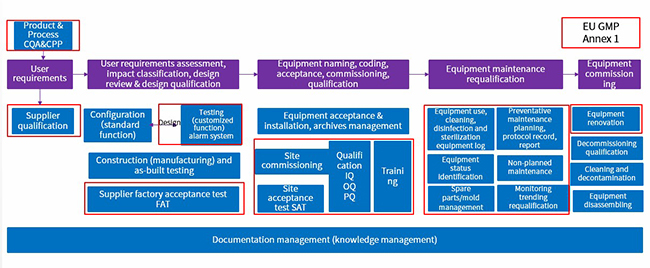
Keynotes are described as follows:
1. Product and process CQA&CPP — sterile medicinal products, characteristics of promoting microbial growth, hazards, etc.
2. Supplier qualification — audit and qualify suppliers of facilities and equipment for sterile medicinal products based on risk assessment.
3. Alarm system — equipment monitoring requirements should be defined in user requirements specifications, process and equipment alarms should be verified and evaluated for trends; the frequency at which alarms are assessed should be based on their criticality (with critical alarms reviewed immediately).
4. FAT, commissioning, SAT, qualification — all commissioning and qualification activities are performed as planned, with the scope and extent based on risk assessment.
5. Training — ensure manufacturers of sterile medicinal products master equipment principle and identify existing risks.
6. Equipment cleaning, disinfection and sterilization — CIP/SIP, remove any residue or debris that may compromise the effectiveness of detergents and disinfectants; minimize chemical, microbial and particle contamination of the products.
7. Equipment status identification — reduce human errors, ensure normal use of equipment.
8. Management of spare parts/molds — for aseptic processes, direct and indirect product contact parts should be sterilized.
9. Preventative maintenance — perform the maintenance at periodic intervals to reduce the probability of equipment failure and continuously ensure the performance of facility and equipment.
10. Unplanned maintenance — corrective actions are taken when the equipment fails in operation or when potentials of equipment failure are found, the aseptic processing environment should be protected during maintenance.
11. Monitoring trend analysis, requalification — facilities and equipment are qualified regularly to ensure the system to always stay in the state of control.
12. Equipment renovation — continuous improvement is based on new product and process requirements or regulatory compliance.
The equipment lifecycle management also involves calibration, deviation, change and other GMP activities, all of which should be incorporated into the quality management system for unified management.
References:
1. EC EudraLex - Volume 4 GMP Guidelines Annex 1 Manufacture of Sterile Medicinal Products, 2022
2. Guidance of Good Manufacturing Practices for Drug (2nd edition): Quality Management System, 2023
3. Guidance of Good Manufacturing Practices for Drug (2nd edition): Premises, Facilities and Equipment, 2023







 Search
Search 中文
中文







