A Diagram Illustrating the Requirements for the Entrusted Party in the Contract Manufacturing
News & Insights2024-07-08
Innovation is one of the main drivers for the pharmaceutical industry in China, as elsewhere. Since the revised Drug Administration Law took effect in 2019, the MAH system has been implemented nationwide in China. It is necessary for pharma companies pursuing development in the Chinese market to understand the MAH system. Based on the practical situation of implementing the MAH system in recent years, the biggest challenge for MAH holders lies in how to fulfill the main responsibility of ensuring the quality and safety of the drug throughout its life cycle while delegating production to CDMOs.

In pharmaceutical contract manufacturing, the entrusting party often lacks the power to effectively supervise and manage the quality management system of the entrusted party. What requirements does the entrusted party typically need to meet? We have created a diagram outlining the essential points for the entrusted party to comply with the MAH quality management system. This diagram is intended to assist you in navigating the process, and we hope it proves to be a valuable reference.
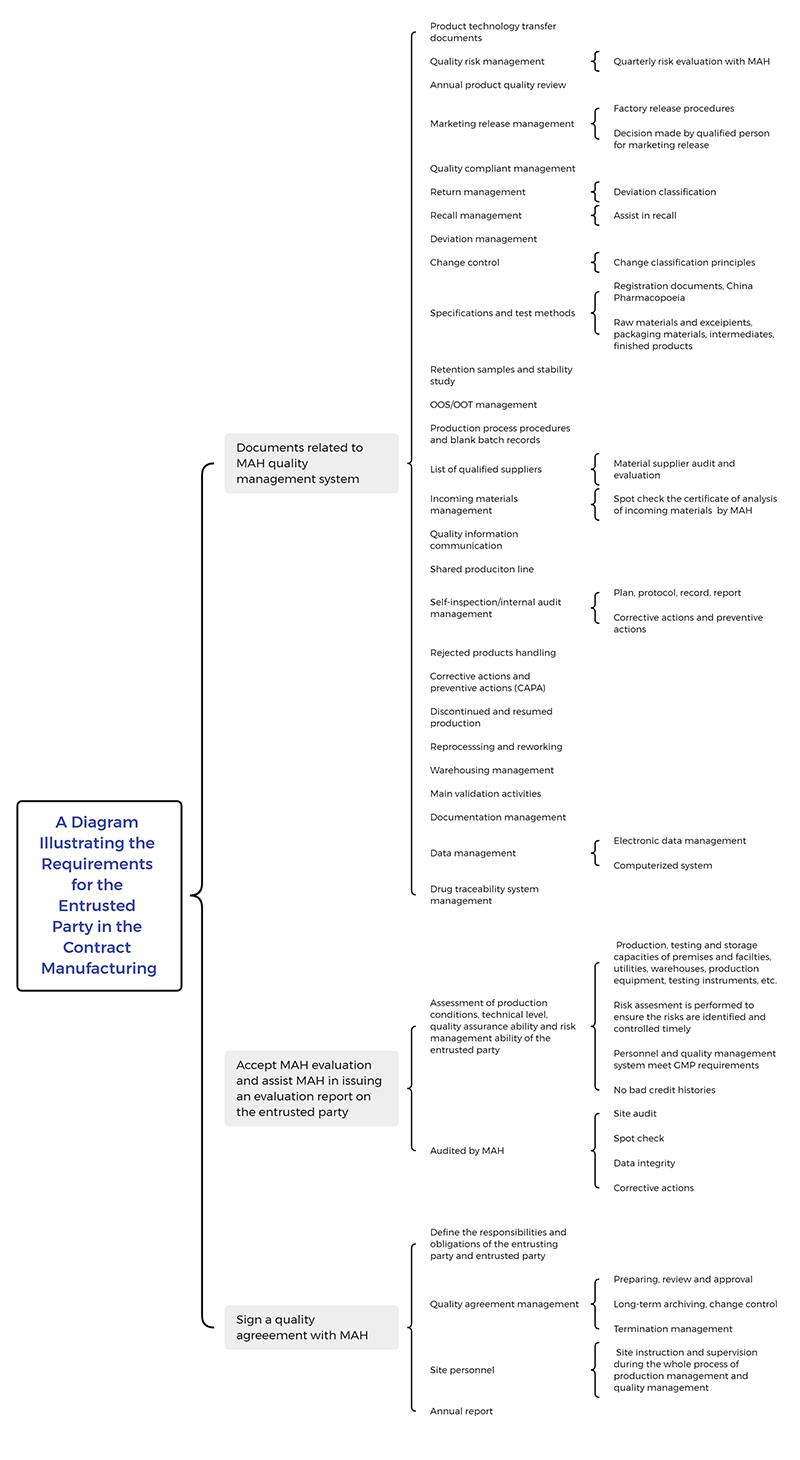
 A Diagram Illustrating the Requirements for the Entrusted Party in the Contract Manufacturing.pdf
A Diagram Illustrating the Requirements for the Entrusted Party in the Contract Manufacturing.pdf







 Search
Search 中文
中文







