EU GMP Annex 1 - Airflow Pattern Studies
News & Insights2024-05-30
The main risk to product quality in the production environment of sterile products lies in the contamination of microorganisms and particles in the production environment. Every effort should be made to minimize the risk of microbial and particle contamination to the product.

Clean airflow is the main way to remove particles generated during drug production, and a good airflow pattern is an important guarantee for a clean environment. When coming to airflow pattern studies, the following key areas should be considered:
Grade A critical areas, grade A air supply equipment (such as isolators, RABS and biosafety cabinets), as well as cleanrooms (especially background rooms in critical areas).
Critical equipment operation areas with critical pressure gradients, such as RABS door opening, junction of open isolators, crossing areas with air locks, mouse holes from capping to packaging, mouse holes from tunnel to filling, and airlock door opening.
Airflow pattern in dry heat sterilization area, freeze dryer loading and unloading area.
The development and implementation of airflow pattern studies
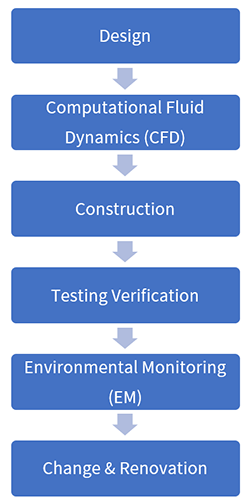
Scope of airflow pattern studies
Activities performed at rest and in operation, including machine not running, machine running at no load, and machine running at load with human intervention.
For grade B background airflow visualization studies, personnel should conduct simulation operation at the grade A area to observe whether the airflow can achieve purging effect.
Intervention by aseptic operation.
Any intervention with airflow, such as environmental monitoring, sampling and rejection, which are considered as fixed interventions and should be tested in airflow pattern studies.
Elements of airflow pattern studies
Sufficient airflow pattern studies are essential to protect products, reduce environmental contamination risk, and create a compliant pharmaceutical environment for producing high-quality sterile drug products and realizing a high sterility assurance level.
Our services
AUSTAR Compliance Consulting Service provides airflow visualization studies for many manufacturers of sterile products, such as vaccines, biological products, blood products and ADC products. If you want to learn more, please feel free to contact us at info@austar.com.hk







 Search
Search 中文
中文







