GMP Design Review Based on EU GMP Annex 1
News & Insights2024-04-17
Introduction
The revised EU GMP Annex 1 Manufacture of Sterile Medicinal Products (which has already been adopted by PIC/S and WHO) illustrates that regulatory authorities expect the pharmaceutical industry to strengthen the protection of sterile medicinal products by adopting newer and better designs and technologies. "Design" is a high-frequency word in EU GMP Annex 1, with requirements from multiple dimensions such as design principles, designer qualifications and designed items.

Design principles
As stated in Section 2 Principle of EU GMP Annex 1, the design principles are as follows:
2.1 The manufacture of sterile products is subject to special requirements in order to minimize risks of microbial, particulate and endotoxin/pyrogen contamination.
2.2 QRM priorities should include appropriate design of the facility, equipment and processes, followed by the implementation of well-designed procedures, and finally application of monitoring systems as the element that demonstrates that the design and procedures have been correctly implemented and continue to perform in line with expectations.
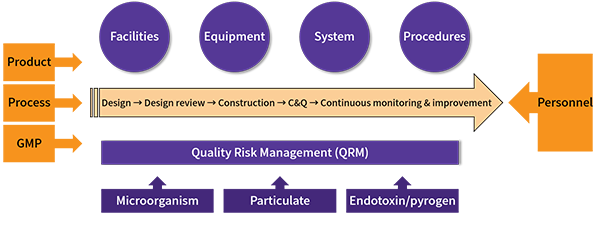
As illustrated in the design principles:
Three inputs: product, process, GMP
One reference basis: quality risk management
Combined with advanced science and technologies to ensure that the manufacturing facilities of sterile medicinal products during the life cycle can prevent contamination from microbial, particulate and endotoxin/pyrogen.
GMP design review
Design principles have already clarified that the manufacturing facilities of sterile medicinal products should be designed in accordance with product attributes, process properties and GMP requirements. Therefore, the outputs of the design should be subject to design review to ensure compliance with the requirements of the revised EU GMP Annex 1.
Design review refers to a planned and systematic review of specifications, and designs, as well as design development and continuous improvement throughout the whole lifecycle of the manufacturing system. It is to evaluate deliverables against standards and requirements, identify problems and propose required corrective actions.
The outputs of different design phases (i.e., conceptual design, basic design and detailed design) are reviewed specifically based on EU GMP Annex 1. It is documentary evidence that the design and facilities comply with relevant requirements of EU GMP Annex 1 for the design basis and the GMP requirements of the project and ensure that the manufacturing facility of sterile medicinal products is designed to minimize risks to product quality and patient safety.
Our services
At AUSTAR, We are committed to providing GMP design review consultancy based on EU GMP Annex 1 for sterile products, biological products, blood products, ADCs,etc., to ensure the projects meet EU GMP standards and smoothly progress to the construction phase.
References
1. EU GMP Annex 1 Manufacture of Sterile Medicinal Products _20220825
2. ASTM E2500-20 Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment







 Search
Search 中文
中文







