Complex Injectable —— Microsphere Formulation Preparation System (A)
News & Insights2023-06-30
Complex injectables are novel parenteral products that require cutting-edge technology for innovative drug delivery, including drug loading systems (DLS) and drug delivery systems (DDS). Compared to conventional injectables, these new drug molecules serve the advantages of targeted delivery, long-acting effects, and sustained and controlled release. Therefore, reducing the dosing frequency and significantly improving the clinical efficacy which is making them a focal point for pharmaceutical companies. Complex products are defined as products with complex active ingredients, complex formulations, complex auxiliary carriers and complex equipment. As a result, excellent integration of formulation, process and equipment hardware is required to achieve successful complex injectables development.
Complex injection preparations include liposomal, microsphere, fat emulsion, nanocrystal, albumin nanoparticle, and micelle. The preparation methods and equipment involved in the Microsphere injectable formulations will be further discussed in this article.
1. Overview
Drug-loading microspheres in DDS (Drug Delivery Systems) refer to small spherical or approximate sphere structures formed by dissolving or dispersing drugs in polymer materials, typically ranging in size from 10 to 400μm. The drug-loading principle of microspheres involves the physical embedding or adsorption of APIs (Active Pharmaceutical Ingredients) surface or inside the polymer matrix, while the stability of the polymer ensures sustained release of the drugs.
(1) Principles of Drug Release
The release mechanism of microspheres involves the controlled diffusion or degradation of the polymer matrix, which encapsulates the drug. The drug is slowly released as the polymer matrix gradually degrades or erodes over time. This allows for sustained and controlled release of the drug, which can improve therapeutic efficacy and reduce side effects compared to conventional dosing methods.
(2)The Advantage of Microspheres
Security: Polymeric microspheres exhibit slow drug release at the site of administration, maintaining a favorable blood concentration, minimizing fluctuations in blood drug levels, and reducing the incidence of adverse effects. The biodegradable nature of polymeric microspheres allows for degradation into water and carbon dioxide within the human body, thereby enhancing their safety profile.
2. Preparation Method
The preparation of microspheres, which is dependent on the physicochemical properties of the Active Pharmaceutical Ingredient (APIs), commonly employs techniques such as emulsion solvent evaporation, phase separation, spray drying, hot-melt extrusion, and other methods.
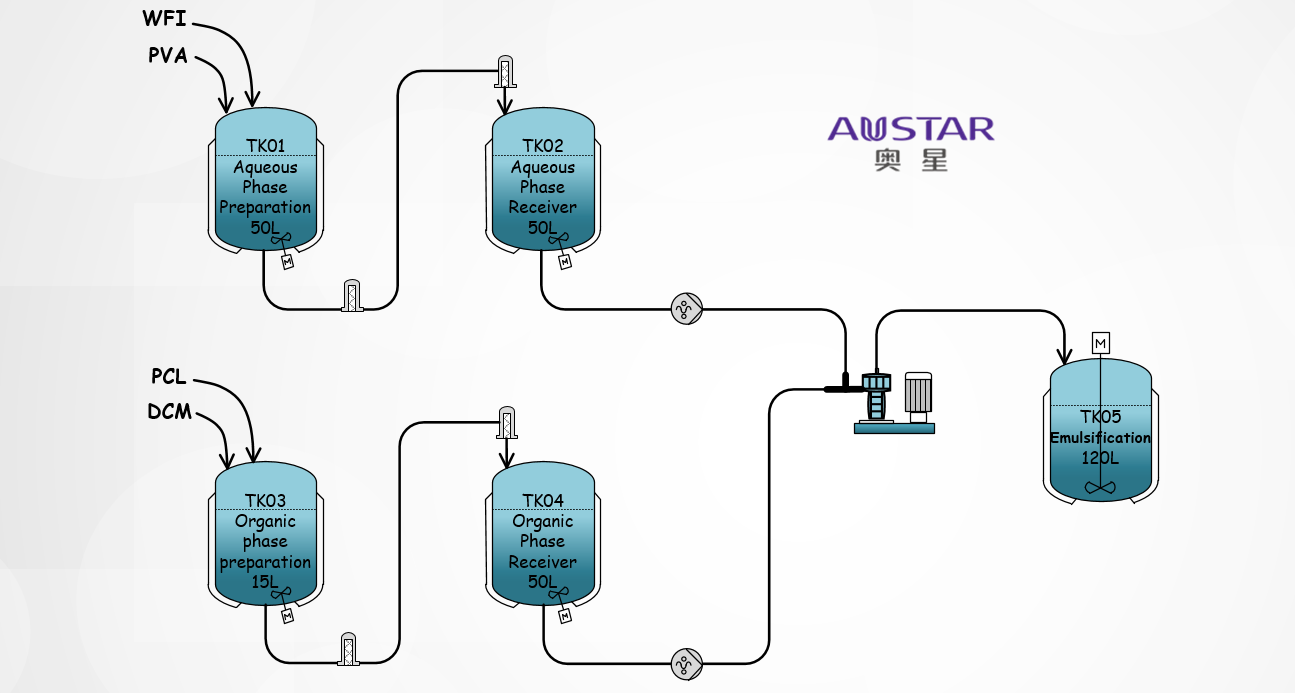
(1) Emulsion solvent Evaporation Method
For lipophilic drugs, the Oil-in-Water (O/W) emulsion method is commonly used for microsphere preparation.
In this method, the lipophilic active ingredient and excipients are dissolved in an organic solvent as the oil phase, while a hydrophilic emulsifier such as polyvinyl alcohol (PVA) is dissolved in the aqueous phase. Under high-Shear conditions, the oil phase is added to the aqueous phase to form O/W emulsion droplets. The organic solvent in the emulsion is then evaporated under physical conditions, resulting in solidification and formation of microspheres as the organic solvent content decreases.
(2) Spray Drying Method
Spray Drying is a method of producing microspheres by spraying liquid feedstock into a hot drying medium, transforming the feedstock into dry powder in the spray drying apparatus.
(3) Hot-melt Extrusion Method
Hot-melt extrusion is a commonly used technique for preparing particles, granules, and implants. Similar to the spray drying method, hot-melt extrusion does not require the dissolution of feedstock materials and allows for direct extrusion of feedstock mixtures upon heating.
3. Polymer Material
Polymeric materials are the critical excipient in microsphere technology, with fundamental requirements of biocompatibility and biodegradability. Therefore, to maintain material stability, it is necessary to avoid exposure to water, acidic substances, alkaline substances, alcohols, and other reagents that may cause product degradation during storage. The storage environment should be sealed, dry, and kept at a low temperature.
Types of polymer materials are commonly used including Poly (D,L-lactide-co- glycolideacid) (PLGA), Poly lacticacid (PLA), Poly(ε-caprolactone) (PCL)







 Search
Search 中文
中文








