Complex Injectable — Liposomal Formulation Preparation System (A)
News & Insights2023-05-24
Complex injectables are novel parenteral products that require cutting-edge technology for innovative drug delivery, including drug loading systems (DLS) and drug delivery systems (DDS). Compared to conventional injectables, these new drug molecules serve the advantages of targeted delivery, long-acting effects and sustained and controlled release. Therefore, reducing the dosing frequency and significantly improving the clinical efficacy which is making them a focal point to pharmaceutical companies. Complex products are defined as products with complex active ingredients, complex formulations, complex auxiliary carriers and complex equipment. As a result, excellent integration of formulation, process and equipment hardware is required to achieve successful complex injectables development.

Complex injection preparations include liposomal, microsphere, fat emulsion, nanocrystal, albumin nanoparticle, and micelle. The Liposomal formulation preparation system will be further discussed in this article.
Liposomes are spontaneously formed closed spherical vesicles composed of concentric bilayer membranes made of phospholipids and cholesterol. Due to the amphiphilic nature of the phospholipids, water-soluble APIs (active pharmaceutical ingredients) are encapsulated within the aqueous compartments, while lipophilic APIs are entrapped within the phospholipid bilayers. Liposomes can generally be classified into Multi-Vesicular Liposomes (MVLs), Large Unilamellar Vesicles (LUVs), and Single Unilamellar Vesicles (SUVs), among others, depending on their size and internal structure.
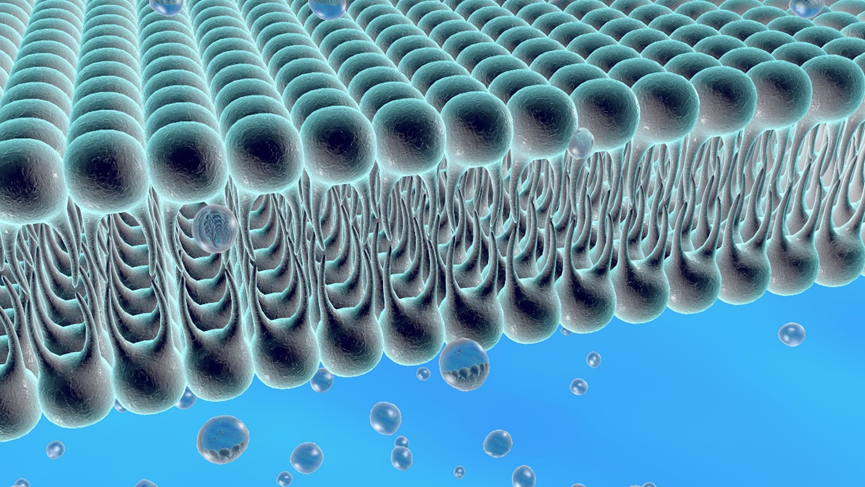
Components of Liposomes
Phospholipids are a class of amphiphilic molecules characterized by hydrophobic tail groups and hydrophilic head groups. The head groups of phospholipid molecules exhibit hydrophilic properties, while their fatty acid tails typically consist of acyl chains and display hydrophobic characteristics.
Sterols have become essential components of cell membranes, imparting bilayer fluidity, permeability, and stability. Many sterol additives are incorporated into liposome structures to enhance the stability of vesicles. Cholesterol is one of the most widely used molecules for improving liposome stability because of its ability to modulate bilayer membrane fluidity.
Preparation Methods for Liposomes
Based on the dynamic characteristics of liposome assembly, various methods are available for the preparation of liposomes, such as thin film hydration, reverse-phase evaporation, solvent injection, microfluidics and extrusion.
Thin-Film Hydration (TFH) Method (Bangham Method)
The thin film hydration method (Bingham) is one of the widely used methods for liposome preparation. This method involves the dissolution of phospholipids in an organic solvent or a mixed solvent system containing organic solvents. Usually, the organic solvent is evaporated to form a lipid film.
Solvent Injection Method
The solvent injection involves first dissolving phospholipids in an organic solvent and then thoroughly mixing the solution with an aqueous medium containing the drug to be encapsulated in liposomes. The lipids arrange as a monolayer at the interface between the organic phase and the aqueous phase, which is a critical step in forming the bilayer structure of liposomes.
Microfluidic Method
The microfluidic method is based on micro emulsification and can be used for the scale-up production of liposomes. In a microfluidic device, lipids can be added in the form of dispersed multilamellar vesicles or as unhydrated lipids dispersed in an organic medium, such as a phosphate buffer or volatile solvent. The microfluidic device pumps fluids at very high pressure through narrow channels. Then, it guides the fluid at high speed through the interaction chamber in the determined microchannel, where it undergoes shear, collision, turbulence, or cavitation effects, thereby affecting effective energy transfer.
Extrusion Method
The extrusion method is used to produce uni-lamellar liposomes of defined size. Multilamellar liposomes are forced through a narrow pore filter under pressure, leading to membrane rupture and release, which causes leakage of the encapsulated material. Hydrated liposome vesicles are forced through a stack of bilayers of polycarbonate membranes with gradually decreasing pore sizes at a specific temperature. To obtain vesicles of the desired size, each bilayer is extruded several times.
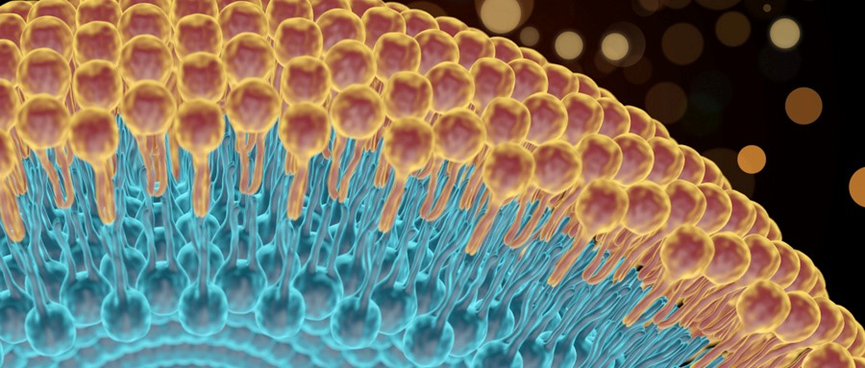
Drug Load for Liposomes
Active Loading
For encapsulation of APIs, pre-made empty liposomes are mixed with concentrated API solution and incubated for a period of time, during which the drug is uniformly distributed in the liposomes through a diffusion process. Due to the highly permeable nature of the phospholipid bilayer to the API diffusion, a high encapsulation rate can be achieved within an appropriate timeframe. Based on the concentration gradient, the drug can penetrate through the phospholipid bilayer into the interior of the liposome until equilibrium is reached between the surrounding medium and the interior of the liposome.
Passive Loading
The use of passive loading technology depends on the ability of the liposomes to encapsulate a specific volume of aqueous phase containing dissolved drug or solute during the formation process.
For hydrophilic APIs, the encapsulation rate by passive loading liposomes is related to the volume of the aqueous phase in which the liposomes are located. For lipophilic APIs, interaction with the phospholipid bilayer determines the encapsulation rate, which depends on the type and concentration of the phospholipids used.







 Search
Search 中文
中文









