Pros and Cons of Unidirectional Flow Facility Layout Design
News & Insights2022-11-08
A pharma facility with a unidirectional flow is the preferred layout for many biopharmaceutical companies specializing in Mab, ADC, vaccines, ATMP and high potent related products. But does this kind of layout really go into achieving the right solution for your business? In this article, we will discuss what a unidirectional flow facility layout design is and why it is essential, the pros and cons of a unidirectional flow facility design, and applicable occasions to understand how unidirectional flow should be.
What is a unidirectional flow facility layout design?
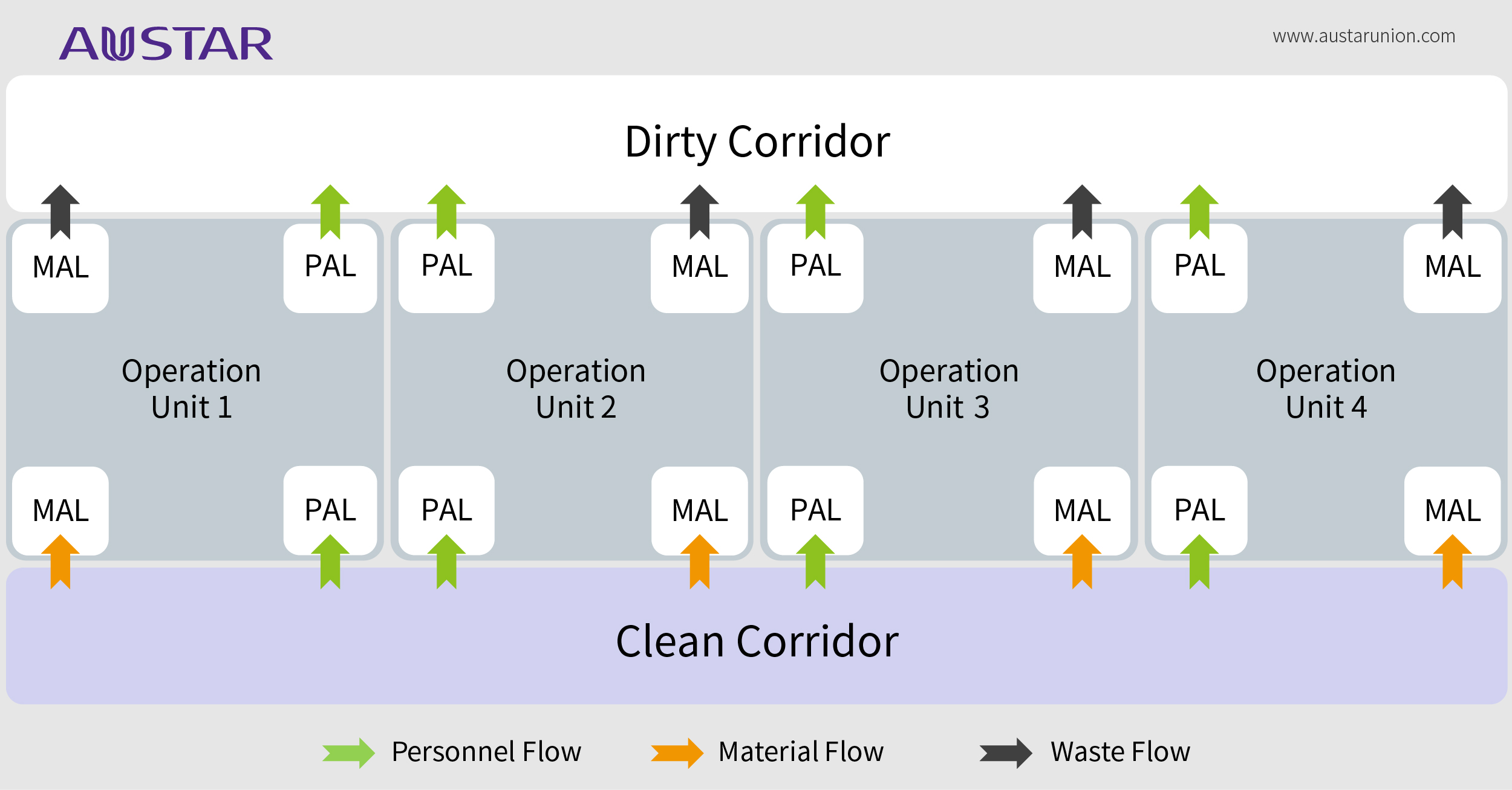
In short words, the unidirectional flow facility layout design is one concept that uses physical segregation strategy to separate clean areas from dirty area through double corridors. This kind of layout is the most effective arrangement for clean and dirty diversion, to better solve the problem of mix-up and cross contamination which GMP concerns.
Considerations for a unidirectional flow facility design:
When considering a unidirectional flow, pharmaceutical process engineers should consider the boundary of the "clean" area and "dirty" area during the design phase.
"Clean" area refers to the area where the critical material (such as materials with high potent, high toxicity, biological activity or easy to cause aerosol and dust contamination, etc.) is not exposed during normal operation, this area will not directly generate contamination and affects the surrounding environment, normally including personnel gowning in, temporary storage area, and solution preparation area, etc.;
"Dirty" area is relative to the “Clean” area, where the critical material is exposed during normal operation, directly generates contamination and affects the surrounding environment, normally including the main operation area, personnel de-gowning out, solid waste temporary storage, inactivation and treatment area, etc.
In some occasions, a unidirectional flow facility layout provides secondary containment. Therefore, in addition to spatial layout, there are several factors you should consider to match it, including air-conditioning partition, supply and exhaust ventilation system, airlock system and differential pressure design while applying unidirectional flow. However, when your workplace or product is protected by primary containment efficiently, or apply closed systems directly, the necessary for unidirectional flow facility design in this case needs to be fully evaluated
Pros and Cons of Unidirectional Flow facility layout Design
As with traditional design schemes, there are pros and cons of a unidirectional flow facility layout design, we’ll review some of these benefits and drawbacks to keep in mind when discussing applicable occasions with unidirectional flow
Pros:
? Through completely controlling flow path for personnel, material and waste, reduce the risk of operation post-crossing, material mix-up and cross-contamination.
? Effectively reduce the area of the high risk operation area, such as the high potent area by separating from a clean area.
? In some occasions, circulating air is adopted in the clean area, and fully fresh air is adopted in the dirty area, which is more conducive to energy saving and consumption reduction.
? For the CDMO factory, it will keep the operating areas with relative independence, which is also beneficial to multi-product production mode.
Cons:
? It increases the total building area of the production workshop or reduces the utilized area of the function room within the limited building area.
? It brings inconvenience to production operations, especially for some production workshops with several operation sequences which will be arranged to perform in dedicated function rooms respectively.
? The frequency of personnel gowning and de-gowning is increased, and the number of clean clothes and washing frequency is also increased correspondingly.
? In some cases, it will increase the number of air conditioning units and occupy more technical room areas.
? Total investment cost is increased.
Applicable occasions with unidirectional flow facility layout design:
According to the above Pros & Cons analysis, this layout concept is more suitable for the following occasions:
? High toxicity or high potent production facility, such as ADC conjugation facility
? High-level animal houses, such as SPF and GF animal houses;
? Production workshops and laboratories with high biosafety levels, such as certain vaccine facilities with high biosafety levels.
? Multi-product and multi-line facility, such as CDMO facility
? Multiple-batch are produced simultaneously within the dedicated operating unit, such as a workshop for cell and gene therapy.
Often Pharmaceutical Good Manufacturing Practice (GMP) and Lean objectives are counter-intuitive. It is crucial to design a facility correctly at the conceptual stage. The unidirectional flow facility layout design can provide better solutions to mix-up and cross-contamination in comparison to the traditional design scheme. However, there are many factors should be taken into consideration to achieve one safe, compliant, efficient, energy-saving and lean production facility. Therefore It is necessary to rely on a professional engineering design team to make a reasonable design based on various factors on site and clear user requirements during the design phase,







 Search
Search 中文
中文






